How Long After Stoppingomeprazole Before I Feel Normal Again
- Use of PPIs in New Zealand
- When, and how, is it appropriate to prescribe a proton pump inhibitor?
- When can y'all consider stopping treatment with a PPI?
- How safe are proton pump inhibitors?
- References
In this commodity
In this article ![]()
View / Download pdf version of this article
Use of PPIs in New Zealand
The treatment of symptoms caused past gastric acrid dates backs to the aboriginal Greeks, who used coral pulverisation (calcium carbonate) to convalesce dyspepsia.ane During the 1970s and '80s H2-receptor antagonists, e.m. ranitidine, were introduced. This was followed by the introduction of proton pump inhibitors (PPIs), which were fifty-fifty more effective in reducing gastric acrid production. PPIs take now largely superseded H2-receptor antagonists, resulting in an improved quality of life for many patients. Their effectiveness, however, has too led to PPIs being used more widely in primary care than almost whatever other medicine.
In 2013, there were 428 dispensed prescriptions for omeprazole for every 1000 registered patients, making it the third most widely prescribed medicine in New Zealand.2 The number of patients prescribed PPIs in New Zealand has increased steadily over the by five years (Effigy 1). In 2013, $iv.28 one thousand thousand was spent in the New Zealand health sector on omeprazole capsules lone; over one-quarter of this was for omeprazole xl mg capsules, the highest dose conception available.3
Which PPIs are available in New Zealand?
In New Zealand there are 3 fully subsidised PPIs available on the Pharmaceutical Schedule: omeprazole, lansoprazole and pantoprazole. These 3 medicines are also available for buy in limited quantities, without prescription, as a "Chemist's shop but" medicine. Rabeprazole is available unsubsidised, with a prescription. Patients should exist asked about whatever employ of not-prescription medicines before acid suppressive medicines are prescribed.
 Refer to the New Zealand Formulary for further details on these medicines: world wide web.nzf.org.nz
Refer to the New Zealand Formulary for further details on these medicines: world wide web.nzf.org.nz
Omeprazole, lansoprazole and pantoprazole are indicated for:4
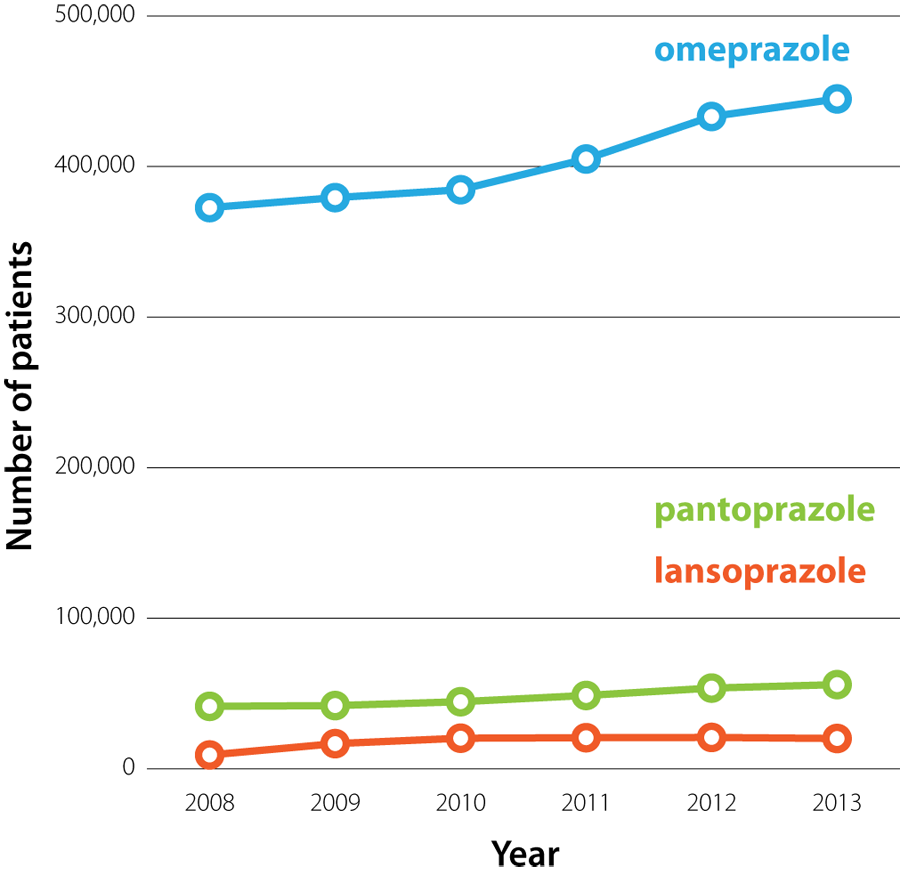 |
| Figure 1: The number of patients who were dispensed omeprazole, pantoprazole or lansoprazole from a community chemist's in New Zealand (2008 – 2013).3 |
- Treatment of gastro-oesophageal reflux disease (GORD), including Barrett'southward oesophagus
- Prevention of NSAID-associated duodenal or gastric ulcers (omeprazole and pantoprazole only)
- Treatment of benign duodenal and gastric ulcers
- Eradication of Helicobacter pylori (as function of a combination regimen with antibiotics)
- Treatment of Zollinger-Ellison syndrome (omeprazole and pantoprazole only)
The PPIs available in New Zealand have a like efficacy when given at recommended doses; e.g. a meta-assay found there was no divergence in the comparative effectiveness of PPIs in healing oesophagitis.5 The availability of three subsidised PPIs means that if a patient experiences adverse effects with one PPI, another tin can be trialled. It too allows for choices in formulation, eastward.chiliad. pantoprazole is bachelor in small tablets that may be preferable for patients who take difficulty swallowing.
When, and how, is it appropriate to prescribe a proton pump inhibitor?
When initiating PPI treatment information technology is helpful to talk over with patients what the expected duration of treatment is likely to exist. This reinforces the bulletin that treatment will non keep indefinitely, unless the indication remains, and is likely to make later discussions about dose adjustment and treatment withdrawal easier.
For most patients an advisable starting regimen is omeprazole xx mg, once daily (depending on the indication).4 In some patients, treatment may demand to be increased to 40 mg, daily, if symptoms are non able to exist controlled, merely starting treatment initially with omeprazole 40 mg, in one case daily, is rarely indicated in a chief care setting. Over fourth dimension, and again depending on the indication for treatment, the dose of PPI may exist able to exist reduced, due east.g. from 20 mg to 10 mg omeprazole, daily, or inverse to "as needed" dosing, if adequate control of symptoms is accomplished.
Northward.B. Before prescribing a PPI information technology is important to consider a patient'southward take a chance factors for gastric cancer, as PPI utilize tin mask the symptoms of this malignancy. The incidence of gastric cancer increases substantially after age 55 years, and a decade before in people of Māori, Pacific or Asian descent.seven
The pharmacology of proton pump inhibitors
PPIs are prodrugs, i.e. they are inactive when administered and undergo conversion to an agile form in vivo.6 PPIs are acid labile and are therefore formulated with an enteric coating to protect them from deposition in the acidic environs of the tum. In one case they take passed through the tummy and the enteric coating has dissolved in the small intestine, PPIs are absorbed into the blood where they have a relatively short plasma one-half-life of 1 – one.v hours.ane The effect of PPIs extends well beyond this half-life, because the active metabolite binds irreversibly to the H+/G+-ATPase proton pump of parietal cells. This prevents the ship of acidic hydrogen ions into the gut lumen for ten – 14 hours.6 The acid-suppressing effect of PPIs takes at least v days to reach a maximal effect.i However, this effect is not accented; fifty-fifty at loftier doses approximately one-quarter of proton pumps in each parietal cell will remain active.6
Gastrin is the hormone that stimulates parietal cells to release gastric acid. When PPI inhibition of gastric acid production occurs, gastrin release is increased to compensate for the decreased acidity of the breadbasket. Recently, several studies have suggested that when PPIs are withdrawn the torso will keep to produce gastrin at higher up pre-treatment levels, causing an effect referred to equally rebound acid secretion.half-dozen
Gastro-oesophageal reflux disease
Proton pump inhibitors are indicated in the treatment of suspected or confirmed GORD. The treatment regimen depends on the severity of symptoms and the likelihood of the patient developing complications. PPIs tin can be used to:1, 8
- Plant a diagnosis of GORD via empiric treatment over several weeks
- Provide "every bit needed" relief of symptoms in patients with milder forms of GORD
- Provide daily symptom relief for patients with more severe symptoms
When managing patients with balmy GORD, information technology is important that the patient and clinician both agree that the regimen will be regularly reviewed, with the goal of treatment existence lifestyle control of symptoms with minimal reliance on medicines. The lowest effective dose of PPI should exist used for the shortest possible time.
 For further data, see: "Update on the direction of gastro-oesophageal reflux illness".
For further data, see: "Update on the direction of gastro-oesophageal reflux illness".
Non-steroidal anti-inflammatory drug (NSAID)-associated ulcers
PPIs are indicated for the prevention and treatment of NSAID-induced erosions and ulcers in at run a risk patients (run into below), and are oftentimes prescribed to care for NSAID-induced dyspepsia.4 PPIs should be taken daily, rather than "as needed", to prevent NSAID-related agin effects because ulceration or bleeding of the alimentary canal tin can occur in the absence of dyspepsia.ix
Take chances factors for gastrointestinal adverse effects, e.one thousand. perforations, ulcers and haemorrhage, associated with NSAID use include:ix, 10
- Historic period over 65 years
- Previous adverse reaction to NSAIDs
- The utilize of other medicines that may exacerbate any gastrointestinal adverse furnishings, e.g. anticoagulants, antiplatelets and corticosteroids
- A history of cardiovascular disease
- Liver illness
- Chronic kidney disease
- Smoking
- Excessive booze consumption
Many of these risk factors are also contraindications to the utilize of NSAIDs.
A PPI is advisable for patients with any of the above risk factors, who are taking NSAIDs long-term. Patients should be advised to written report whatsoever gastrointestinal symptoms (e.g. heartburn, black stools) which may indicate that an erosion or ulcer has occurred.9 Also consider checking the patient'south haemoglobin level subsequently 1 month of NSAID handling.nine
For ulcer prevention, the recommended regimen is omeprazole xx mg, in one case daily, for the duration of NSAID treatment.4 To treat NSAID-associated duodenal or gastric ulcers the recommended regimen is omeprazole 20 mg, once daily, for four weeks, which may exist continued for a further four weeks if required.iv Pantoprazole is an alternative in both regimens if omeprazole is non tolerated.4 Lansoprazole is not indicated for ulcer prevention in patients taking NSAIDs, but tin can be used for treatment of ulcers.four
 For further data see: "Non-steroidal anti-inflammatory drugs (NSAIDs): Making safer treatment choices", BPJ 55 (Oct, 2013).
For further data see: "Non-steroidal anti-inflammatory drugs (NSAIDs): Making safer treatment choices", BPJ 55 (Oct, 2013).
Eradication treatment for H. pylori
Proton pump inhibitors are recommended for the eradication of H. pylori as part of a triple handling regimen. For example, a 7 twenty-four hours course of:4
- Omeprazole 20 mg, twice daily; and
- Clarithromycin 500 mg, twice daily; and
- Amoxicillin one g, twice daily (or metronidazole 400 mg, twice daily, if allergic to penicillin)
Other regimens using different dosing intervals or other PPIs, e.g. lansoprazole, tin likewise be used (see NZF for farther information).
Confirmation of eradication of H. pylori after a triple treatment regimen is not required for the majority of patients. A test of cure may be considered in patients with a recurrence of symptoms, a peptic ulcer complication or those with of import co-morbidities.11
 For further information, see: "The changing face of Helicobacter pylori testing", BT (May, 2014).
For further information, see: "The changing face of Helicobacter pylori testing", BT (May, 2014).
When can yous consider stopping treatment with a PPI?
Many patients taking PPIs crave long-term treatment and withdrawal of the PPI will exist inappropriate, east.1000. patients with Barrett'southward oesophagus. In other patients, e.chiliad. with a history of severe erosive oesophagitis, withdrawal of PPIs should only be considered after word with an advisable specialist. Still, in each exercise population there will be some patients for whom it is appropriate to reduce the dose of the PPI they are prescribed, e.m. from twenty mg omeprazole, once daily, to 10 mg omeprazole, one time daily, or switching to "equally needed" dosing. For patients taking PPIs long-term the need for ongoing treatment should be reassessed at every consultation.
The patient's expectations when the PPI was first prescribed will play a large part in their acceptance of the suggestion to reduce their PPI exposure. There is no clear prove as to what the all-time regimen for withdrawing PPI treatment is, merely in general, downwardly dose titration should be considered when symptoms are under command.vi For example, a patient is prescribed twenty mg omeprazole, daily, for four to six weeks to manage symptoms of GORD. The patient responds to treatment and their symptoms resolve. The dose is so reduced to 10 mg, daily, for 2 weeks, and then treatment is stopped. The patient is given a prescription for 20 mg omeprazole to use "as needed" if symptoms return.
Advise patients almost the possibility of rebound acrid secretion
Rebound acid secretion can occur when PPIs are withdrawn; one report found that more than 40% of asymptomatic patients experienced dyspepsia one week after completing a four week handling course of pantoprazole.12 Serum markers propose that acrid secretion one week following cessation of PPI treatment tin be significantly increased higher up pre-treatment levels. This should render to normal within two weeks.12
The symptoms caused by rebound acid secretion, e.thou. gastro-oesophageal reflux, are the same as those that would be an indication for PPI treatment, therefore a reinforcing loop can be formed where initial treatment creates the need for further treatment. The possibility of rebound acrid secretion should be discussed with patients then they can be prepared for this when withdrawing from PPI treatment.
Medicines that comprise both an antacid and an anti-foaming amanuensis, e.g. Mylanta P oral liquid, Acidex oral liquid, Gaviscon Double Strength tablets are probable to exist the almost effective treatment for rebound acrid secretion. Aluminium hydroxide tablets can besides be effective. Any of these products can be prescribed as "rescue" medication and provide reassurance to patients if symptoms return.
 For farther data see: "Managing gastro-oesophageal reflux disease (GORD): an update".
For farther data see: "Managing gastro-oesophageal reflux disease (GORD): an update".
How prophylactic are proton pump inhibitors?
The rate of adverse furnishings associated with PPI treatment is relatively low. Nonetheless, given that each practice is likely to have many patients taking PPIs, clinicians demand to exist aware of the potential risks. These risks should be discussed with patients, and the need for periodic monitoring considered in those at increased risk.
All three subsidised PPIs available in New Zealand can cause headache and gastrointestinal adverse effects, e.g. nausea, airsickness abdominal pain, flatulence, diarrhoea or constipation.4 The gastrointestinal agin effects of PPIs can be mistaken for symptoms of GORD, sometimes resulting in increased doses of PPI beingness prescribed. Less frequently, PPI use is associated with dry oral fissure, peripheral oedema, dizziness, slumber disturbances, fatigue, paraesthesia, arthalgia, myalgia, rash, pruritus and interstitial nephritis.4
PPIs are non known to be associated with an increased chance of foetal malformations in humans (Pregnancy Chance Category B3).iv PPIs are therefore considered safety to use during pregnancy, nonetheless, other medicines should exist used where possible. A reasonable approach for pregnant women who crave acid suppressive medication is to trial antacids (eastward.g. calcium carbonate, alginate formulations) or ranitidine (Pregnancy Risk Category B1) start and if these medicines are not constructive, consider prescribing a PPI.
Higher doses of PPIs should be avoided in patients with moderate to severe liver illness because decreased metabolism may cause the medicine to accumulate (come across NZF for details).4
The risk of infection is increased
Gastric acid suppression with PPIs increases the take a chance of infection with gastrointestinal or respiratory pathogens, although the absolute risk to almost patients remains depression. The higher gamble is thought to be due to a reduction in the effectiveness of the "acrid wall" stomach barrier. This allows feasible pathogens to travel upwards or downward the gastrointestinal tract and besides colonise the lower airways.
Where possible, consider delaying the initiation of PPIs in patients with an increased gamble of infection, e.g. an older patient with a family fellow member who has influenza, patients who are taking antibiotics or travelling to countries where at that place is a high take chances of enteric infection.6 Information technology is not known if there is any do good to temporarily stopping treatment in patients who are already taking PPIs, during periods when they are at an increased risk of infection.
In a meta-analysis of 12 studies involving nearly three 000 patients, information technology was found that acid-suppressing treatment increased the take chances of C. difficile infection. This risk was increased 1.7 times with once-daily PPI apply and two.four times with more than once daily apply.13 Six studies institute a greater than iii-fold increased risk of Salmonella, Campylobacter and Shigella infection in patients taking PPIs.13
In some other report of over 360 000 people, it was found that PPI apply was associated with an increased risk of pneumonia, and the take a chance increased with increasing dose of PPI.14 The incidence charge per unit of pneumonia in people taking a PPI was two.45 per 100 person-years, compared to 0.6 per 100 person-years in people non taking a PPI.fourteen Another study found that the likelihood of patients developing pneumonia was increased five-fold during the first week of PPI handling, but decreased later this, falling to 1.three-fold increased risk after patients had been treated for three months or more.15 This effect may be explained past patients presenting with the early on symptoms of pneumonia being prescribed a PPI.6
Malabsorption of nutrients may occur
Acrid in the gut increases the solubility of elements, east.g. calcium and fe, from insoluble salts and makes poly peptide-spring vitamins, east.k. vitamin B12, available for absorption. It has therefore been suggested that gastric acid suppression may decrease assimilation of some nutrients and lead to an increased prevalence of conditions related to malabsorption. However, this clan is controversial. In most cases, patients tin exist reassured that a balanced diet, including essential elements and minerals (e.thou. calcium, iron, folate, magnesium) is acceptable to address this take a chance.
Long-term PPI use has been associated with a small increase in fracture risk. However, the New Zealand Medicines Adverse Reactions Committee (MARC) noted that the clan between PPI apply and fracture risk in the majority of studies was pocket-sized and does not warrant any regulatory action at this time.sixteen A study of more than 15 000 instances of osteoporosis-related fractures institute that after five years of PPI use patients had an increased adventure of hip fracture (adapted odds ratio = 1.62), and the chance increased further when treatment was continued for seven years (adjusted odds ratio = 4.55).17 Patients taking PPIs for more than than seven years also had an increased risk of not-hip fractures (adapted odds ratio = i.92).17
An increased chance of osteoporosis should be considered in post-menopausal females who are taking PPIs long-term, especially if they take other take chances factors, eastward.g. a family history of osteoporosis or long-term corticosteroid use. Stepping down PPI handling to the lowest effective dose, or prescribing "as needed" treatment, if appropriate, may reduce this gamble.
Astringent hypomagnesaemia has been associated with the utilize of PPIs, in a limited number of patients, which resolved when PPI handling was withdrawn.eighteen In 2012, Medsafe advised that hypomagnesaemia, and maybe hypocalcaemia, were rare adverse furnishings of PPI use. Omeprazole, 20 – twoscore mg per 24-hour interval, was the dosage most ofttimes associated with these deficiencies.19 Magnesium is known to affect calcium homeostasis by deceasing parathyroid hormone secretion and decreasing the response of the kidney and the skeleton to parathyroid hormone.19
Patients with a history of excessive alcohol employ, who are taking a PPI, have an increased chance of developing hypomagnesaemia due to the additive effects of chronic ethanol exposure on metabolic function. The use of diuretics, ciclosporin or aminoglycosides with PPIs increases the risk of hypomagnesaemia occurring. Symptoms of hypomagnesaemia are non-specific and may include muscle cramps, weakness, irritability or defoliation.
Routine testing of magnesium levels in patients taking PPIs is by and large not recommended. Withal, if a patient has been taking a PPI long-term and they present with unexplained symptoms that are consistent with hypomagnesaemia, consider requesting a serum magnesium level. Increased dietary intake of magnesium rich foods, due east.g. nuts, spinach or wheat, or magnesium supplementation may exist sufficient to improve serum magnesium levels while continuing the PPI. For some patients the PPI will need to be stopped; if the indication for using the PPI is strong, a re-challenge while monitoring magnesium can exist undertaken.
 For further data come across: "Hypomagnesaemia with proton pump inhibitors" BPJ 52 (April, 2013).
For further data come across: "Hypomagnesaemia with proton pump inhibitors" BPJ 52 (April, 2013).
Vitamin B12 deficiency has been associated with the use of PPIs in older patients.18 Several short-term studies take shown that PPIs decreased the absorption of vitamin B12 from food.18 In older patients with poor nutrition, who are taking PPIs long-term, consider testing vitamin B12 levels periodically.18
Hyponatraemia has been associated with the employ of PPIs in a very pocket-sized number of patients.20 Hyponatraemia, however, is a relatively common occurrence in older people, many of whom are likely to exist taking PPIs.
Astute interstitial nephritis has been associated with PPIs
Prior to June 2011, the Heart for Adverse Reactions Monitoring (CARM) had received 65 notifications of interstitial nephritis linked to PPI use.21 Interstitial nephritis tin result in permanent kidney damage.vi Symptoms and signs suggestive of interstitial nephritis include: fever, rash, eosinophilia, angst, myalgia, arthralgia, weight loss, altered urine output, haematuria or pyuria and/or loftier blood pressure.21 NSAIDs are well known for their nephrotoxic potential and their use should increase suspicion of interstitial nephritis in patients with these symptoms. Other risk factors that would increase the suspicion of interstitial nephritis include the employ of β-lactams, e.g. penicillins or cephalosporins, sulphonamides and diuretics, or the presence of infection or immune and neoplastic disorders.21 If interstitial nephritis is suspected, request urine microscopy and renal role tests. The patient should be referred to a Nephrologist for assessment. To confirm a diagnosis of interstitial nephritis a renal biopsy is required.
Interactions with other medicines
Concerns of a possible interaction between omeprazole and clopidogrel are unlikely to be clinically significant. MARC assessed the prove of an interaction betwixt PPIs and clopidogrel and concluded that while there was evidence that PPIs may affect clopidogrel activity ex vivo, the available bear witness suggested that this would non translate to clinically significant adverse outcomes.22 At that place is no need to switch treatment for patients who are already taking a PPI and clopidogrel. However, if considering prescribing a PPI at the same fourth dimension as clopidogrel and so pantoprazole is the recommended choice. Pantoprazole is known to have less of an inhibitory consequence on the CYP2C19 enzyme compared with omeprazole or lansoprazole.23
PPIs can crusade a minor increment in the anticoagulant effect of warfarin or a decrease when the PPI is stopped. Patients taking warfarin should have their INR measured more than frequently post-obit the initiation, or discontinuation of PPIs to ensure they practice non experience a clinically meaning interaction.8
"Take-home" points about PPIs
- Review all existing patients taking PPIs long-term and appraise whether the indication for treatment remains and whether the dose of PPI can be reduced
- When new patients are started on PPIs, discuss the expected duration of treatment and accept a programme for stepping down or stopping treatment
- In most situations, patients do non need to exist started on PPI treatment in primary care with xl mg omeprazole, daily (or equivalent)
- There are few patients who should exist taking 40 mg, omeprazole, daily long-term
- Consider whether "as needed" apply would be more advisable for patients than taking PPIs daily
- Ensure patients are prepared for rebound acid secretion which may occur when PPI treatment is withdrawn; antacids can be used as a "rescue medicine"
Acknowledgement
Thanks to Dr Jason Colina, Gastroenterology Clinical Leader, Southern DHB for expert review of this article.
References
- Dutta U, Moayyedi P. Direction of reflux-related symptoms. Best Pract Res Clin Gastroenterol 2013;27:387–400.
- bpacnz. 2013 Almanac Exercise Written report - Pharmaceutical and Laboratory Test Utilisation. 2013. Available from: www.bpac.org.nz/Written report/2013/Nov/annRep.aspx (Accessed Jun, 2014).
- Ministry building of Health (MoH). Pharmaceutical collection. 2014.
- New Zealand Formulary (NZF). NZF v23. 2014. Available from: www.nzf.org.nz (Accessed Jun, 2014).
- Wang West-H, Huang J-Q, Zheng Thousand-F, et al. Caput-to-head comparing of H2-receptor antagonists and proton pump inhibitors in the handling of erosive esophagitis: a meta-analysis. World J Gastroenterol 2005;11:4067–77.
- Yang Y-X, Metz DC. Safety of proton pump inhibitor exposure. Gastroenterology 2010;139:1115–27.
- New Zealand Guidelines Group. Suspected cancer in chief care: guidelines for investigation, referral and reducing disparities. NZGG, 2009. Available from: world wide web.health.govt.nz (Accessed Jun, 2014).
- Gastroenterological Guild of Australia (GESA). Reflux disease: Gastro-oesophageal reflux affliction in adults. Victoria: GESA 2011. Available from: world wide web.gesa.org.au/files/editor_upload/File/Professional person/Reflux_Disease.pdf (Accessed Jun, 2014).
- Mean solar day RO, Graham GG. Not-steroidal anti-inflammatory drugs (NSAIDs). BMJ 2013;346:f3195.
- Hawkey CJ, Langman MJS. Non-steroidal anti-inflammatory drugs: overall risks and management. Complementary roles for COX-two inhibitors and proton pump inhibitors. Gut 2003;52:600–8.
- New Zealand Guideline Grouping. Management of dyspepsia and heartburn. Prove-based best practice guideline summary. NZGG, 2004. Bachelor from: www.health.govt.nz (Accessed Jun, 2014).
- Niklasson A, Lindström L, Simrén Grand, et al. Bitchy symptom development subsequently discontinuation of a proton pump inhibitor: a double-bullheaded placebo-controlled trial. Am J Gastroenterol 2010;105:1531–vii.
- Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol 2007;102:2047–56.
- Laheij RJF, Sturkenboom MCJM, Hassing R-J, et al. Risk of community-acquired pneumonia and employ of gastric acid-suppressive drugs. JAMA J 2004;292:1955–60.
- Gulmez SE, Holm A, Frederiksen H, et al. Use of proton pump inhibitors and the chance of community-acquired pneumonia: a population-based instance-command study. Arch Intern Med 2007;167:950–v.
- Minutes of the 143rd Medicines Adverse Reactions Committee meeting: Risk of osteoporosis and fractures associated with proton pump inhibitor handling. Medsafe, 2013. Available from: www.medsafe.govt.nz/profs/adverse/Minutes143.htm#3.4 (Accessed Jun, 2014).
- Targownik LE, Lix LM, Metge CJ, et al. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ 2008;179:319–26.
- Ito T, Jensen RT. Association of long-term proton pump inhibitor therapy with bone fractures and effects on absorption of calcium, vitamin B12, atomic number 26, and magnesium. Curr Gastroenterol Rep 2010;12:448–57.
- Medsafe. Omeprazole and risk of hypomagnesaemia. Prescriber Update, 2013. Bachelor from: www.medsafe.govt.nz/profs/PUArticles/OmeprazoleJune2010.htm (Accessed Jun, 2014).
- Liamis M, Milionis H, Elisaf M. A review of drug-induced hyponatremia. Am J Kidney Dis Off J Natl Kidney Found 2008;52:144–53.
- Medsafe. Proton pump inhibitors and interstitial nephritis. Prescriber Update, 2013. Available from: www.medsafe.govt.nz/profs/puarticles/protonpumpsept2011.htm (Accessed Jun, 2014).
- Medsafe. Update: PPIs and Clopidogrel Interaction. Prescriber Update, 2014. Bachelor from: world wide web.medsafe.govt.nz/profs/PUArticles/March2014UpdatePPIsAndClopidogrel.htm (Accessed Jun 2014).
- Drepper MD, Spahr 50, Frossard JL. Clopidogrel and proton pump inhibitors-where do nosotros stand in 2012? World J Gastroenterol 2012;xviii:2161–71.
Source: https://bpac.org.nz/bpj/2014/june/ppi.aspx
0 Response to "How Long After Stoppingomeprazole Before I Feel Normal Again"
Post a Comment